Innovative ‘living drug’ treatment approved and successfully used for adults (> 15 years) is awaiting approval to offer new hope to children battling refractory B-cell lymphomas and acute B-cell lymphoblastic leukemia.
Mumbai: Made-in-India CAR T-cell therapy, an innovative medical technology for cancer treatment, has shown significant results for refractory B cell leukemias and B cell lymphoma patients in India, with trials yielding positive results. This procedure has recently also been successfully used to treat two adult patients (> 15 years) in India at Apollo Hospitals, Navi Mumbai.

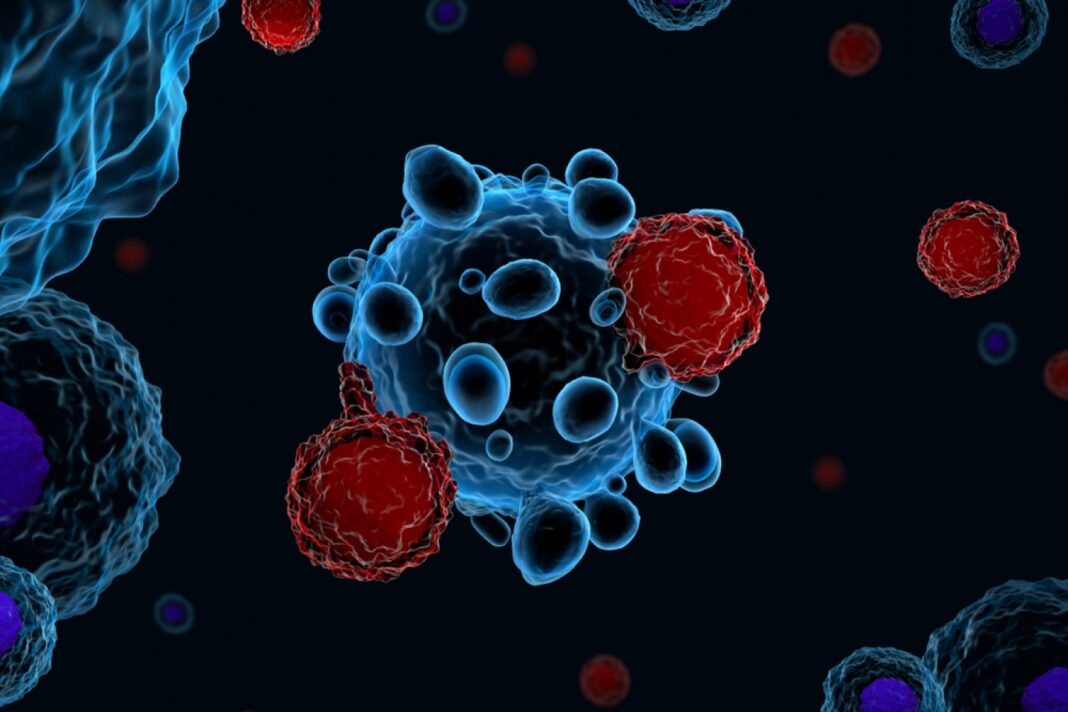
The made-in-India CAR T-cell therapy is a ground-breaking immunotherapy that uses the patient’s own genetically modified immune cells to target and destroy cancer. The therapy could also be beneficial for treating rheumatological and immunological diseases in the future once it receives approval from the government and health departments.
In The U.S., Already a Success

At just six years old, Emily Whitehead from the United States faced a life-threatening recurrence of leukemia that resisted 16 months of chemotherapy. Her parents, Tom and Kari, enrolled her in a clinical trial for a new immunotherapy, CAR T-cell therapy, which had never been tested on children. The treatment successfully put Emily’s cancer into complete remission. By June 2012, at age 7, she was discharged, and nearly 1,000 people gathered in her town of Philipsburg, Pennsylvania, to celebrate her victory over leukemia.
Dr. Punit Jain, DM, Hematology, Apollo Hospitals, Navi Mumbai, said, “CAR T-cell therapy is rewriting what is possible for patients with blood cancers who have relapsed after conventional treatment. This therapy offers hope for a complete cure in patients with aggressive or treatment-resistant cancers. The therapy has also shown positive results for children too, suffering from B-cell leukemia in ongoing trials at Tata Hospitals, and once approved by the relevant authorities, it should be available for a wider audience as well. Since children have better immunity than adults, they can be treated and cured faster, with longer-lasting effects.”
Dr. Jain further mentioned that CAR T procedures has shown a 40-50% survival rate among adult patients with B-cell leukemia and lymphoma treated with CAR T-cell therapy, a significant milestone considering very poor outcomes for such refractory diseases.
Mr. Arunesh Punetha, Regional CEO – Western Region, Apollo Hospitals, said, “These procedures require not only skilled clinicians and trained staff but also a dedicated unit with strict infection control protocols. We are committed to ensuring that patients have access to the latest therapies.”
How CAR T-cell Procedure Works
CAR T-cell therapy is a ground-breaking immunotherapy that uses the patient’s own genetically modified immune cells to target and destroy cancer. Immune cells (called T-cells) are extracted from the patient’s blood and re-engineered in the lab so that they express modified connectors on their surface called Chimeric Antigen Receptors (CARs). These CARs help the immune cells recognize and attack the cancer in the blood. Before receiving the infusion, the patient undergoes a course of chemotherapy to help the modified cells work effectively. CAR T-cell therapy is then administered.